一级标题
一级标题
Avoid your inquiry is delay response, please enter your WhatsApp/Skype along with the message, so we can contact you at the very first time.
We will reply you within 24 hours. If for urgent case, please add WhatsApp/WeChat:
Warning: Undefined variable $public in /www/wwwroot/lvfertilizer.com/wp-content/themes/hyhadmin/header.php on line 350
Warning: Trying to access array offset on value of type null in /www/wwwroot/lvfertilizer.com/wp-content/themes/hyhadmin/header.php on line 350
,. Or call
Warning: Undefined variable $public in /www/wwwroot/lvfertilizer.com/wp-content/themes/hyhadmin/header.php on line 350
Warning: Trying to access array offset on value of type null in /www/wwwroot/lvfertilizer.com/wp-content/themes/hyhadmin/header.php on line 350
directly.
Your plants look weak. The leaves are yellow. You know they need food. But what food do they need? This is a big problem for farmers and gardeners. You need strong plants to get a good crop. Weak plants mean lost money and less food. The solution is often a good fertilizer.
One of the best is Ammonium Sulphate. This guide will tell you all about it.
The ammonium sulphate formula is (NH4)2SO4.
This simple formula holds the key to healthy plants. It is an important inorganic salt. It gives plants two things they need most. It gives them nitrogen. It gives them sulfur. Let’s look closely at this helpful chemical compound.
The formula (NH4)2SO4 looks hard. But it is easy to understand. It is made of two parts.
First, we see (NH4). This is the Ammonium cation (NH4+). A cation is a part with a positive charge. Think of it like the plus (+) side of a battery. The little 2 outside the () means there are two of these ammonium parts. So, we have two positive charges. This part gives plants the nitrogen they need to be green.
Next, we see SO4. This is the Sulfate anion (SO4^2-). An anion is a part with a negative charge. Think of it like the minus (-) side of a battery. It is made of one Sulfur (S) atom and four Oxygen (O) atoms. It has a double negative charge. This part gives plants sulfur. Sulfur helps plants use the nitrogen and make proteins. It is a key part of protein synthesis.
These two parts are held together by an ionic bond. The two positive ammonium parts balance the one double-negative sulfate part. They stick together to make a stable salt. It is a type of double salt.
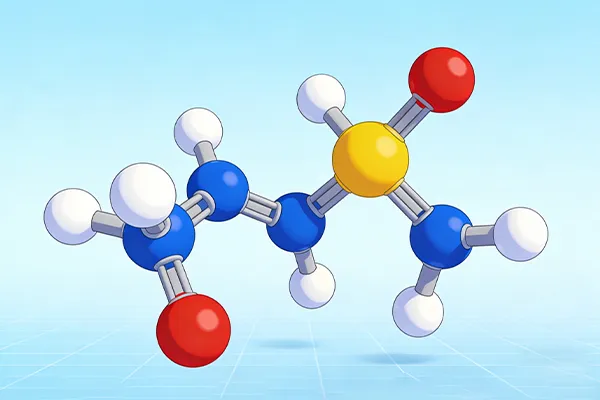
The Structure of (NH4)2SO4
Why is this white, crystalline solid so good for plants and other uses? Its properties are the reason. Understanding them helps you see why it is a top choice.
| Property | Description |
| Formula | (NH4)2SO4, also known as Diammonium sulfate or Sulfuric acid diammonium salt |
| Molar Mass | 132.14 g/mol |
| Appearance | White crystals or granules. It has no smell. In nature, it is found as the mineral Mascagnite. |
| Nitrogen Content | 21%. This is a high amount of nitrogen for plants. |
| Sulfur Content | 24%. This is a great source of sulfur, another key plant nutrient. |
| Solubility in Water | Very high. It is a water-soluble fertilizer. This means it dissolves easily in water. Plants can drink it right up. It is a good solute. |
| Solution pH | An aqueous solution is acidic. This makes it a great soil acidifier. It helps treat alkaline soil treatment. |
| Nature | It has a hygroscopic nature. This means it can pull water from the air. |
| Melting Point | It has a high melting point. It breaks down at temperatures over 235°C. This is its decomposition temperature. |
This useful compound is used in many ways, from the farm to the factory.
This is the biggest job for ammonium sulphate. Plants get sick without enough food. Their leaves turn yellow. They do not grow big. This is a big problem. Ammonium sulphate solves this by providing crop nutrition.
For the best fertilizer for crops, many farmers trust a good ammonium sulphate fertilizer.

Agricultural Fertilizer
Sometimes, you need to spray for weeds. But the spray does not always work well. The water can be hard. The weeds can be tough. This is a problem. Ammonium sulphate helps. When you mix it in, it makes the weed killer work better. It is a top agricultural adjuvant and herbicide spray adjuvant.
Did you know it is in your food? The Food and Drug Administration (FDA) says it is safe. In the food world, it is called E number E517. It works as a food additive. It is an acidity regulator in food. It is also a dough conditioner. It makes bread dough stable. It is a yeast nutrient, helping bread rise perfectly. This is a key part of Food Science.
In a lab, scientists need to study things like proteins. But proteins are very small. They are mixed up with other things in a cell. This is a problem for a scientist. The solution is to use ammonium sulphate. In biochemistry, it is used for protein precipitation. This is a method called salting out. It helps scientists with protein purification and enzyme purification. This lets them study DNA, RNA, and how living things work. It is a very common laboratory chemical and analytical reagent. It can also be used to make a buffer solution.
Fire is a big danger. Some materials burn too easily. Ammonium sulphate helps make them safer. It is used in flame retardant sprays and fire extinguisher powder. It makes it harder for things like wood, textile products, and paper to catch fire. This is an important part of Material Science.
Making ammonium sulphate is a simple chemical reaction. The main way is a chemical synthesis that mixes two things:
When they react, they make a precipitate.
The equation is: 2NH3 + H2SO4 → (NH4)2SO4
It is also made as a byproduct. For example, it is a byproduct of caprolactam production. Caprolactam is used to make nylon. This makes it an important industrial chemical. It is also used in leather tanning and the textile industry for dyeing and printing.
Talk is good, but numbers are better. Data shows just how important ammonium sulphate is. The worldwide market is growing fast. This is because we need more food for more people.
| Study Focus | Crop(s) | Key Findings & Statistics |
| Maize Yield Comparison | Maize | Using a mix of urea and ammonium sulphate boosted crop yield by 10.5% compared to only urea in one study. |
| Soil Acidification | Sugarcane | Long-term use of ammonium sulphate made the soil more acidic, which can be good for certain crops. The soil pH dropped from 5.0 to 4.1 in just 3.5 years. |
| Rice Production | Rice | Studies showed ammonium sulphate worked just as well as urea for growing rice. |
| Coffee Yield | Coffee | Ammonium sulphate worked better than urea for coffee plants when no mulch was used. |
| Tomato Yield | Tomato | The right amount of nitrogen from ammonium sulphate gave the biggest and best tomatoes. |
These studies show that choosing the right fertilizer matters. It changes how well your crops grow. A good nutrient management plan is key for sustainable agriculture.
You see how good ammonium sulphate is. But a bigger problem is finding a good supply. Who can you trust? You need a partner who makes high-quality fertilizer every time. You might need a special mix just for your farm. A generic product may not work. This can lead to wasted money and a poor harvest.
The solution is an experienced fertilizer factory. Shandong Lvfeng Fertilizer Co., Ltd. is a professional fertilizer maker. We started in 1999. For over 25 years, we have helped farmers all over the world.
We have 5 modern production lines. We make 150,000 tons of NPK compound fertilizer and 120,000 tons of ammonium sulphate every year. We make both caprolactam and steel grade nitrogenous fertilizers. Our products provide the perfect NPK ratio for your crops.
We offer more than just one product. We are an expert npk fertilizer supplier. Our products include:
We are your partner in growth. We offer full OEM/ODM services. We can make the exact fertilizer compounds you need.

Choosing the Right Fertilizer Partner
Safety is always important. Is this chemical safe to use? Yes, it is safe when you are careful.
You should always read the Material Safety Data Sheet (MSDS). It can be a skin irritant and an eye irritant. The dust can be an inhalation hazard.
Here are some safety tips:
With these simple steps, it is very safe to handle. Its benefits for agriculture and industry are huge, with very low risk.
Q1: What is the common name for (NH4)2SO4?
A: The common name is ammonium sulphate. Some people say ammonium sulfate. It is also called ammonium sulfide by mistake sometimes, but that is a different chemical.
Q2: Does ammonium sulphate dissolve easily in water?
A: Yes, it is a great soluble fertilizer. Its high water solubility is why it works so fast in the soil. It is a great water soluble nitrogen fertilizer.
Q3: Is ammonium sulphate acidic or basic?
A: It is an acidic salt. Using it will lower the pH of your soil over time, which can prevent nutrient leaching and reduce volatilization. It affects nitrification and denitrification cycles in the soil.
Q4: What is the NPK rating for ammonium sulphate?
A: The NPK rating is 21-0-0. The npk fertilizer full form is Nitrogen (N), Phosphorus (P), and Potassium (K). This means it has 21% nitrogen and no phosphorus or potassium. It is a high nitrogen fertilizer. But it also has 24% sulfur, which is a bonus nutrient not shown in the NPK. It’s great nitrogen for crops like corn. We also offer nitrogen phosphorus and potassium fertilizer if you need a complete N-P-K formula.